A propagating shock front will often drive an undisturbed gas out of chemical and thermal nonequilibrium. This means that the internal degrees of freedom (such as vibrational and rotational levels of molecules) and the composition of the flow do not adjust itself instantaneously to altered ambient conditions. In other words, nonequilibrium occurs due to different equilibration times of translational and internal modes of atomic and molecular species. Chemical and thermodynamic nonequilibrium conditions are often coupled to each other.
Under nonequilibrium, the internal temperature, if exists, does not correspond to the kinetic energy of translational motion of particles. Moreover, the population of internal states (vibrational, rotational and electronic) departs from equilibrium and from Boltzmann distribution due to onset dissociation/recombination.
In HTGD Laboratory, we implement conservation equations (master equations) for each individual internal state of species in order to describe the abundance of nonequilibrium processes. The master equation approach is one of the most accurate and sophisticated ways to assess chemical and thermodynamic nonequilibrium.
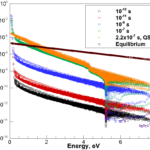
- Populations of approximately 3000 internal states of O2 under shock conditions
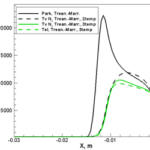
- Nonequilibrium vibrational temperature (green) and translational temperature (black)
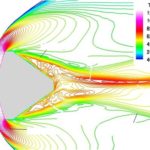
- Bow shock and nonequilibrium flow around reentry capsule