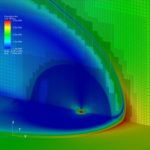
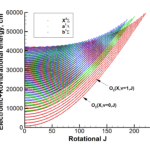
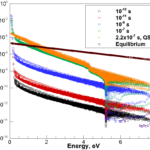
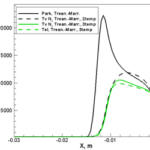
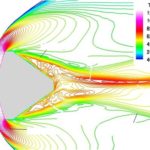
Our interests lay at the coupling of high fidelity models of reactive flows with the state-of-the-art computational methods. Toward this end, we have developed a comprehensive numerical package capable of describing a hypersonic flowfield in the vicinity of a re-entry capsule including the modules of thermodynamics, finite-rate chemistry, multi-dimensional radiation transfer and turbulence.
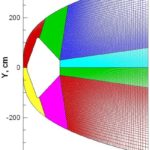
- Example of a multi-block computational grid
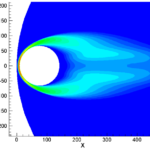
- Computed flowfield around a spherical satellite